
The Core
From H1 to C12
|
This section examines the nuclei between hydrogen and carbon12. The first step is to examination of the alpha particle. The alpha particle is an important nuclear component and is made up of two protons and two neutrons that are arranged in a two-layer, six sided lattice structure which is composed of six up quarks and six down quarks. This section on the core continues examining the remaining stable nuclei between helium 4 and the third nuclear component or first nuclear structure the carbon12 ring. |
||
|
Hydrogen comes in three common varieties: The first two are stable, hydrogen and deuterium, and the third is unstable, tritium. |
||
 |
A=1 The proton is made of two up quarks and one down quark. The two 'UP quarks' are represented by two black ZOME nodes, and the one 'DOWN quark' is represented by a white ZOME node. Interconnections between the quarks in the proton are represented by blue ZOME struts |
|
 |
A=2 |
|
|
When two protons join a positron and a neutrino are ejected to form deuterium |


|
|
|
A=3 IF, Tritium was like an oreo cooky where the proton was the filling it would have a spin of 3/2. However, Tritium has a spin of 1/2 so the two neutrons are joined in either a top and side positon or in two side positions. |
 |
 |
|
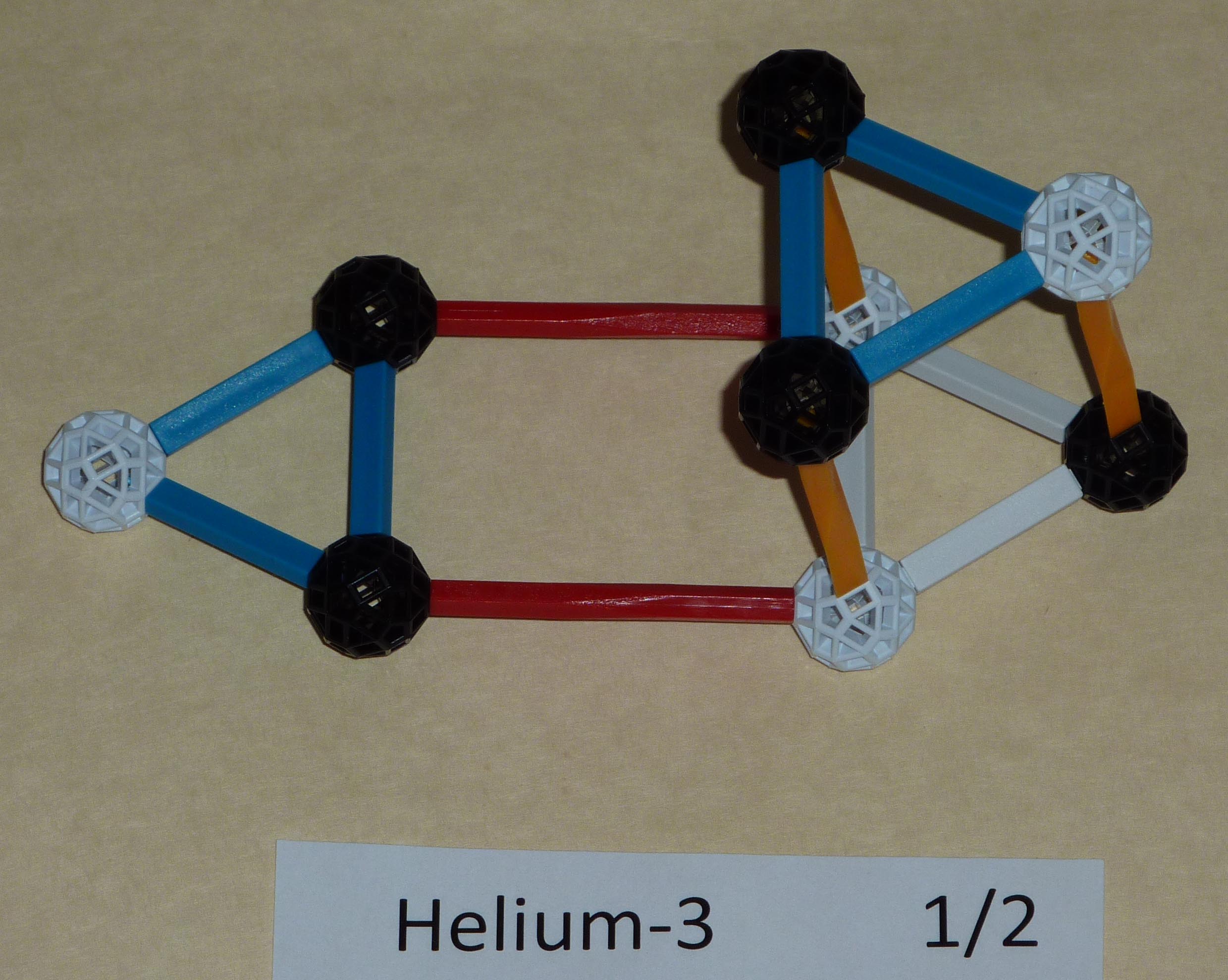
Helium comes in two common varieties: Helium3 - He3 or Hethree |
||

|
A=3 |
|
 |
The possible configurations are the same as tritium just exchanging protons and neutrons. |
 |
|
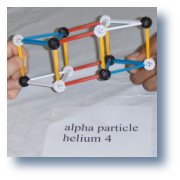
The nucleus of helium4 is also called an alpha particle. |
||
|
A=4 |
|
|
Helium4 If access is not available from the original source this file
is available locally at |
||
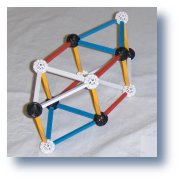 |
Following are some possible ways to visualize the structure of the alpha by visualizing pseudo growth processes: A=4. |
|

|
A=5 |
|
 |
A=6 Lithium6 can be described as two inter-fingered alpha particles where the central deuteron is common to both alpha's. In the figure to the left, the left side alpha is formed using a type one alpha bond and the right side alpha is formed using a type two alpha bond. |
 |
 |
A=7 There is another position where a neutron can fit and the spin will still be 3/2. That position is a branching off from the center proton to from a star shaped structure. |
 |

|
A=8 Why won't or don�t two alphas hold together?
|
|
|
There are three possible ways for the two alphas to attach to each other and each involves four electrical attachment possibilities. Thus, a stable linkage is allusive as the two alphas try the alternate bonding options. |
||



|
||
|
Add a neutron to the mix and a spin of 3/2 and an interesting linkage possibility emerges. |
||


|
A=9
That extra neutron controls the way the two alphas can bond as four deuterons and thus the Be9 nucleus is stable.
|
|
|

|
A=10 |

|

|
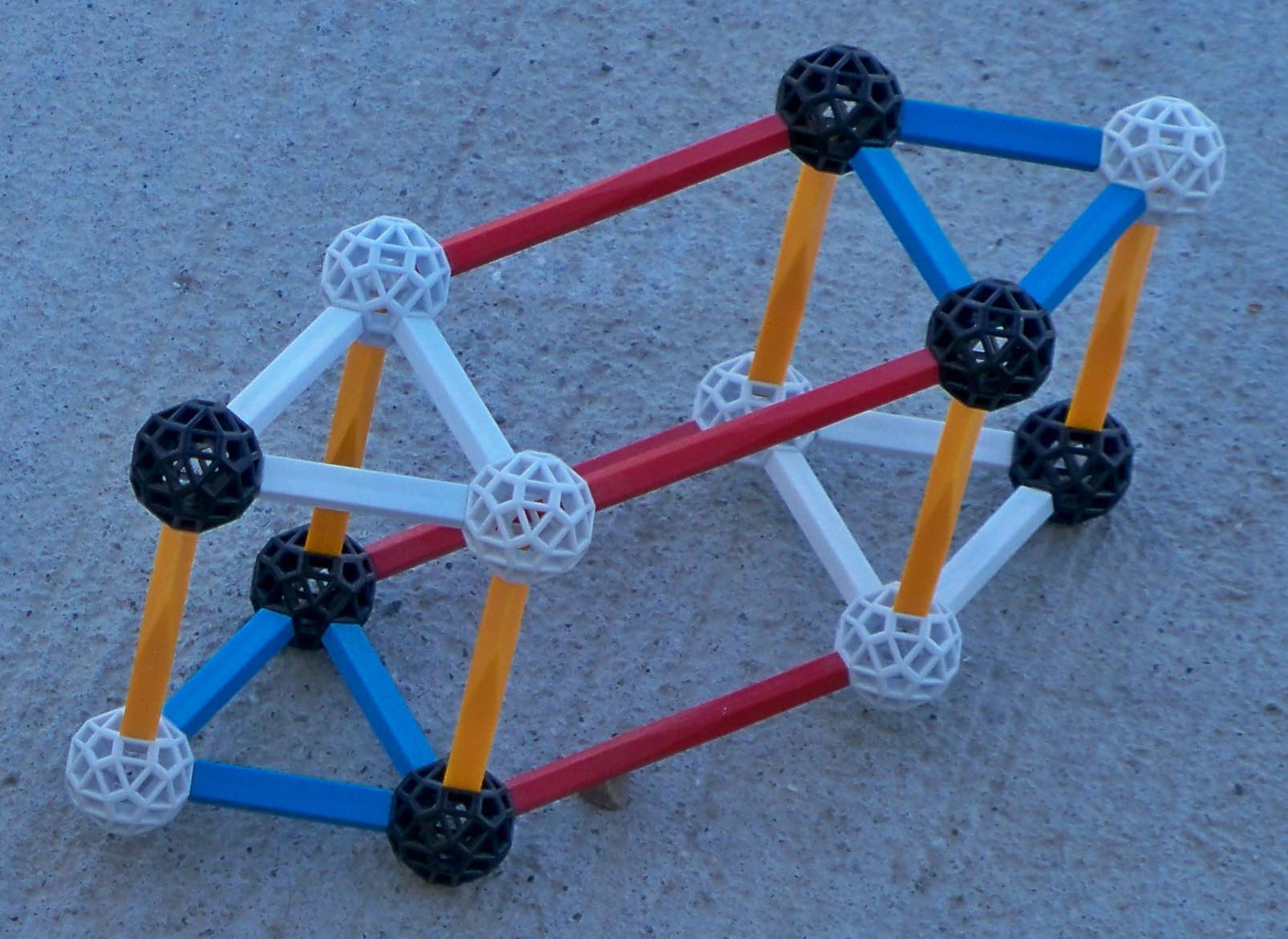
Boron 10 and boron 11 have different basic structures. Thus when a neutron is added to boron 10 the result is NOT boron 11. It is easier for boron 10 and a neutron to split into lithium 7 and helium 4 rather than rearrange to form B11. Review the structure of Li7 (A=7) and compare it to the structure of boron 10. Following is a pictorial representation of neutron absorption by boron 10 which forms boron 11 Temp. The neutron ultimately inducing fission of boron 10 plus a neutron (boron 11Temp) nucleus and why the fission path is more favorable than the path to forming boron 11 
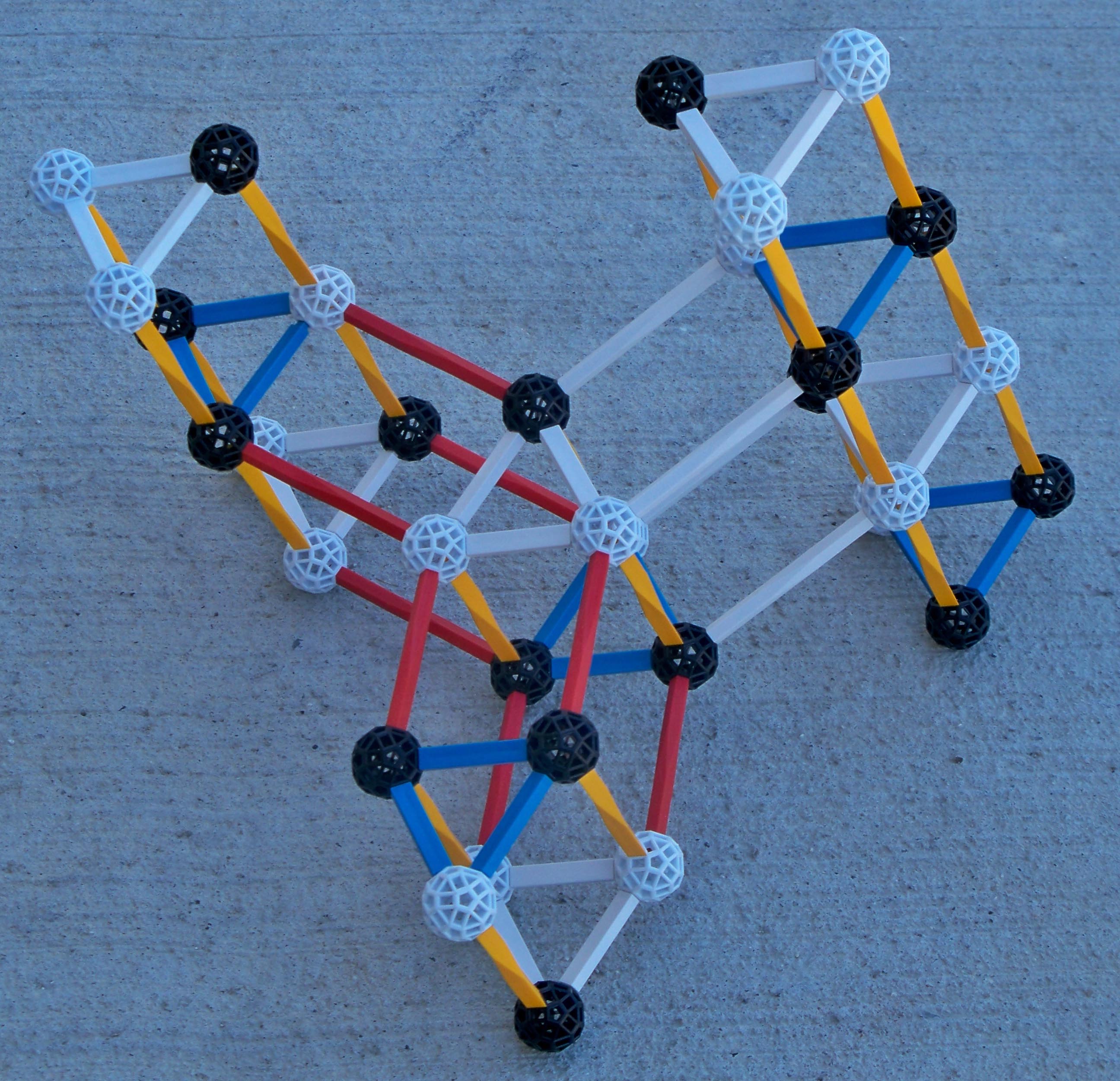
|
The tower or stack of 2 protons and 2 neutrons collapses to form an alpha particle. The sequence is demonstrated below.

|


|
The tower or stack of 2 protons and 2 neutrons collapses to form an alpha particle. The sequence is demonstrated below.


|

|
|
Again compare the nuclear structure of boron 10 with boron 11 and lithium 7. |

BORON11 BORON10 |
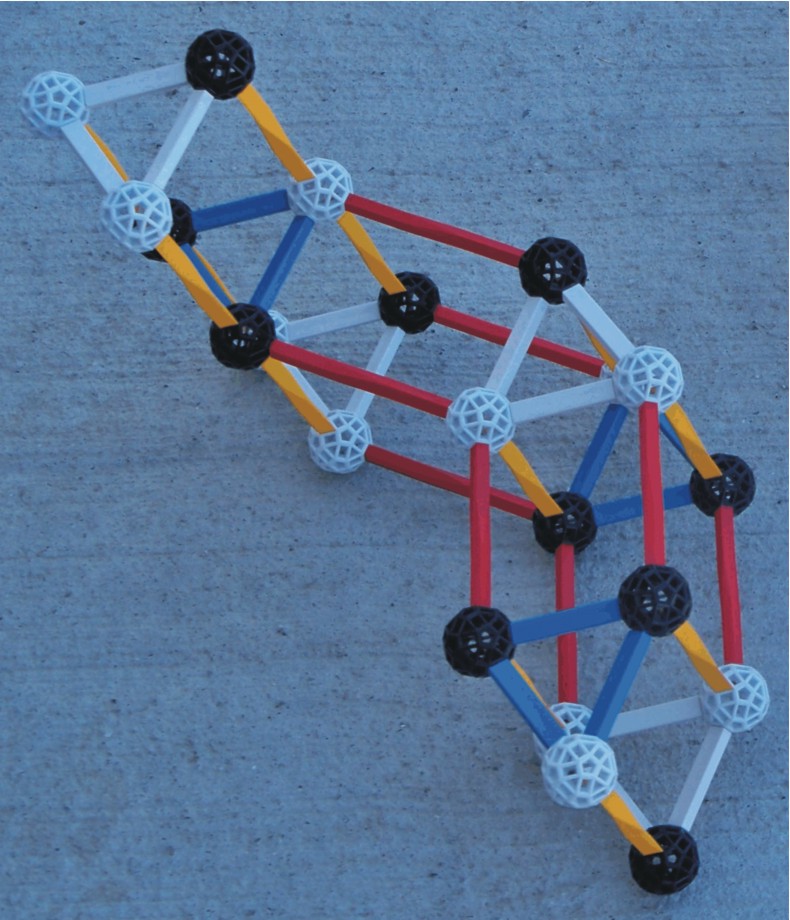
LITHIUM7 |

|
A=11 |
|
|



|
||

|
A=12 |
|
|
Generally a neutron is added before a proton because of the over all positive charge on the nucleus. To this point the nucleus grows one neutron followed by one proton at a time. This continues for two more nuclei while the second carbon ring layer gets started. After the next two nuclei, nucleon additions occurs in pairs. Two, four or six neutrons are first added followed by two protons. For stable nuclei heavier than oxygen the nucleon are always added in even numbers to maintain stability via balance. |
||
