This treatise will introduce you to a complete set of stable nuclei from
hydrogen to bismuth, and then you will step out into the uranium
island and beyond. So join us, there is a lot to study and learn.
You will find that the many mystifying paradoxical aspects of the nucleus
are answered via self-generating facets of the model presented here.
|
|
|
So as previously stated,
The "ah ha" moment came with the realization that protons
and neutron were each composed of three charged particles! Three
particles, no matter how you arrange them, form a flat triangle!
|
Proton
|
Neutron
|

|

|
|
The �ZOME� models show the quarks comprising the proton and
neutron.
- The black nodes represent up quarks.
- The white nodes represent down quarks.
- The nodes connected with blue struts represent protons.
- The nodes connected with white struts represent neutrons.
In other �ZOME� models shown on this page the yellow and red
struts used represent the electrical cross connections of the quarks
that exist between protons and neutrons.
The positive and negative charges of the quarks in two protons
and two neutrons combine to form flattened six sided particles
that are balanced and neutral in all aspects except charge, the
alpha particle.
This changes the perspective of the alpha particle from a tetrahedron
where all the nucleons are equal distance or nearest neighbors, to
where the nucleons are in a plane which makes opposite types nucleons
nearest neighbors and like types of nucleons are second nearest neighbors.
|
|
Alpha particle
|
Combine three alpha particles
to form the Carbon12 ring.
|
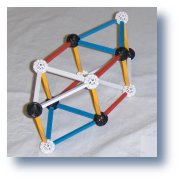
|

|
|
These two structures, the alpha particle and the carbon ring,
dominant the structure of the nucleus.
As the nucleus grows two things will become very obvious.
- First, like nucleons are never nearest neighbors in the nucleus.
- Second, any four adjacent nucleons anywhere in the nucleus always
consists of 2 protons and 2 neutrons and are always in the configuration
of an alpha particle.
Before following the progression of the nucleus in detail, a
quick over view is presented here
of the five major stages that occur in nuclei along the valley of
stability during the building of the nuclear structural lattice. The
following models represent the form the nucleus attains at the end
of each nuclear stage. These models are shown as ZOME constructs.
|
|
|
Three of the carbon rings stacked form “The Core”
of the nucleus. There are semi-completion stages or steps in
building of the core. The first step is the carbon12 ring. The
second step is the completion of the second carbon12 ring to form
magnesium24. The third step is the completion of the third carbon12
ring to form argon36. “The Core” stage is
complete with the nucleus of argon36 shown below.
|

|
|
|
Add neutrons and protons to the out side of the core and “The
Star” is formed. All of the columns developing for the
Star along the outside of the core are capped with neutrons on both ends,
which is how the neutrons help hold the nucleus together against the
building positive charge. Thus these star points or columns are only
five nucleons high while the core is six nucleons high. “The
Star” stage is completed with the nucleus of zinc66 and is shown below
|

|
|
|
The next phase is the Core extension I phase which occurs
in two steps. The first step adds a three proton and three neutron
extension on the ends of the core. The second step also adds another
layer of three protons and three neutrons to further lengthen the core.
The Core Extension I phases are complete with molebdenium96 which is
shown below.

Fill in the gaps between the spikes of the star with alphas and
the “The Loops” are formed. The loops are six
carbon rings inter-fingered with each other and the carbon rings of
the core. The first loop is completed with krypton84. The second
loop is completed with molybdenum98. The third loop is completed
with xenon132. The fourth or last loop of this stage is completed
with neodynium149. Again, the ends of every column still provide
sufficient locations for every extra neutron in the nucleus. At the
end of this stage even the columns forming the core are capped by
neutron. “The Loops” stage is completed with
formation of the nucleus of neodymium149.
|

|
|
|
With the completion of the loops the positive charge has built to
the point that some relief is needed from the building positive
charge, so the next layer on each end of the nucleus forms with
protons located only in an extension of the core. There are no
protons located in the outer loops where they would normally also be
found. There are only neutrons in their expected locations in these
outer loops. These gaps or lack of protons in these outer loops
results in “The Voids” structural stages of the
nucleus. “The Voids” stage essentially involves
all nucleus heaver than neodymium. The gaps occur at the proton
positions in the star radial extension to the core.
“"The Voids"“ can be seen as gaps or voids in
the lattice, which are visible in the next two pictures.
|

|
|
|
|
An intermediate stage is defined following the loops and the voids to
bismuth209 that could be called “The Caps” These
caps are extensions of the core beyond the level of the loops.
|

|
|
|
|
The last stage of nuclear structure is “The
Island” and “The Drift” named because although
there are no stable nuclei in this region, a few long-lived nuclei
do exist. The length of half-lives of the nuclei beyond the voids
depends on how forces and balance of these very large nuclei is
reached. The ?center? most stable nuclei in the island is marked by
its longest lived member, uranium238.
“The Island”
stage reaches completion with 96 protons and ___ neutrons, which is
the element curium.
“The Drift” would probably be
complete with 114 protons and 174 neutrons, which would make element
Ununquadium288. If this stage follows what happens at the end of the other
stages the most stable nucleus should be one or two proton less than
114 or about 112 protons and 172 neutrons for Ununbium284
|

But where is “The End”?
Is it at z = 122 or 124 which have recently been
found in deposits containing thorium? This model indicates
more instability with heavier elements. Even so the model
also indicates that elements with 122 or 124 protons and
also with an even number of neutrons will always be the
most stable isotopes for an element.
|
The data and information depicted in and on this web site are
covered by © “copyright”.
The data and/or information presented in this web site may be
used in part or whole if the user gives credit to the authors and
references this web site.
|











