
Nuclear Periodic Table
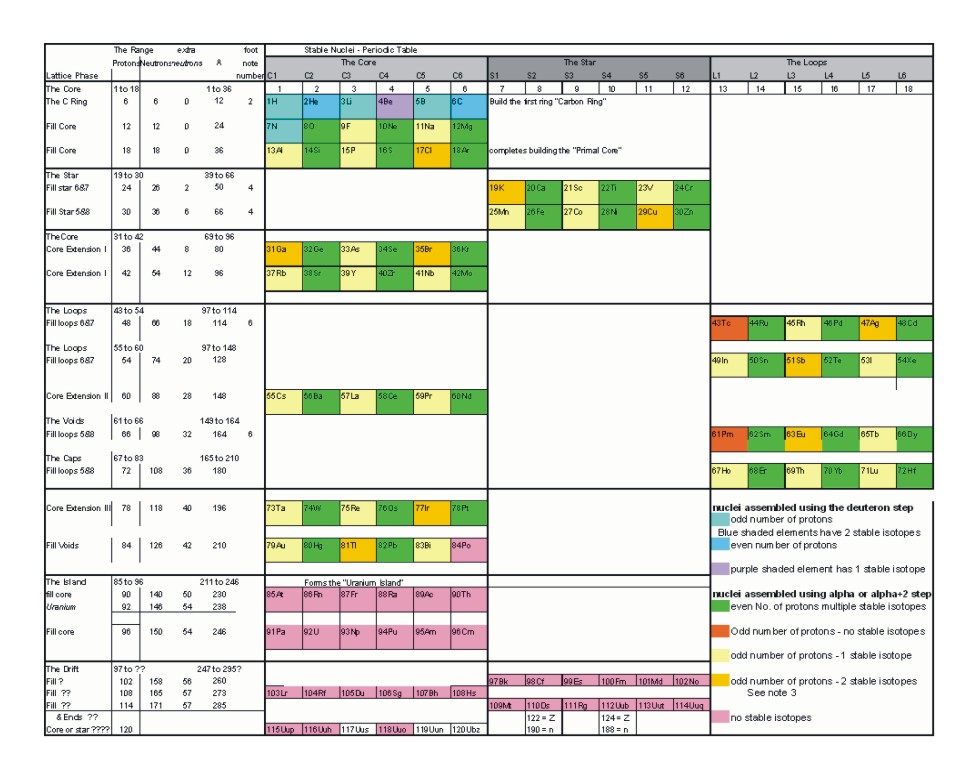
Notes
- The first stage of the nuclear building alternates protons and neutron to maintain electrical balance between the quarks that compose protons and neutrons.
- With the completion of the carbon ring
- When a nucleus has an odd number of protons and has two stable isotopes, the two stable isotopes are separated by an isotope with an odd number of neutrons that is not stable.
- Extra neutrons cap protons on the top and bottom of columns formed by The Star.
- proton and neutron gaps that form no stable isotopes are always odd. This is a result of balance and symmetry with in the lattice structure.
- Extra neutrons again cap protons. Columns formed by The Core, The Star or The Loops are terminated top and bottom by neutrons.
- Satellite stable isotopes always contain an even number of both neutrons and protons. The odd number of nucleons skipped results in sufficient imbalance that those skipped isotopes are unstable.
- When neutrons are added between proton additions, along the path of stability, the neutrons are always added in even increments.
If your looking for a standard periodic table of elememts. There is
a good dynamic one at www.dayah.com/periodic/